methanol production from syngas mass balance
Provided by the Springer Nature SharedIt content-sharing initiative, Over 10 million scientific documents at your fingertips, Not logged in From the previous section, we observed that H2 production peaked at 650 , hence, the effect SGR on syngas composition was determined at STR temperatures of 625 and 650 . PubMedGoogle Scholar. The SCWR process has the disadvantage that substantial hydrogen yields are only obtained at reaction temperatures above 600 , whereas temperatures below 450 favoured the generation of methane [49]. The ICIs company (ed.) Methanol is an interesting fuel for gas turbines engines, due to its potential reduction of NO X and particulate emissions along with the absence of SO 2 emissions. Comput.
Syngas mainly consists of CO and H 2, which can be as raw materials for methanol synthesis using a catalyst in a fixed bed reactor.In recent times, methanol production has been significantly augmented in energy and chemical industries as it is an essential . The syngas ratio was increased by means of a gas treatment process (see next section) before they could be used to synthesize methanol. (2023).
in M85 consisting of 85% methanol and 15% gasoline). WebMost methanol is made from syngas. Renew. Chem Pap 73(11):26192635, Alves HJ, Junior CB, Niklevicz RR, Frigo EP, Frigo MS, Coimbra-Arajo CH (2013) Overview of hydrogen production technologies from biogas and the applications in fuel cells. WebWith the considerations of the complex kinetic mechanisms of the methanol steam reforming using Cu/ZnO/Al2O3 catalyst, in this paper a multiphysics coupling model that integrates the mass, momentum and energy conservation governing equations is proposed to investigate the thermophysical performances of the mid-and-low temperature solar receiver/reactor for The most ideal syngas for the synthesis of methanol has a stoichiometric number (\({S}_{N}\)) of 2 (see Eq. Izbassarov et al. Fuel 307:121821, Sarma SJ, Ayadi M, Brar SK (2016) Biorefinery: general overview. To determine the effects of the key operating conditions which include: STR temperature, steam-to-glycerol ratio (SGR), methanol synthesis pressure, and methanol synthesis temperature, a sensitivity analysis was carried out on the reforming and methanol synthesis section. The residual gas from the PSA-2 was treated further in the PSA-3 after exiting at the bottom. Int J Hydrogen Energy 47(3):14611480, Martn M, Grossmann IE (2017) Towards zero CO2 emissions in the production of methanol from switchgrass. Kyodo, L.Tana, X. Peng, G.Yang, Y. Yoneya, fuel process journals from the. According to the methanation reaction in Equation (6) the mole fraction of CH4 in syngas decreases and that of H2 increases with the increase in temperature. 2126(1), pp. Since the production of methanol takes place in the gaseous phase, it stands to reason that if the reaction temperature is raised, the rate of reaction will also increase, leading to a high amount of products being produced. : Emerging large-scale technologies with industrial potential ; Tzimas, E. methanol synthesis unit was validated with DOE reference production! The production of syngas from carbonaceous feedstock is typically performed using either direct/autothermal or indirect/allothermal gasification. Although the ATR processes can take place at atmospheric pressure, it requires oxygen and higher temperatures to produce effective results [46]. Questo pu essere un buon posto per presentare te stesso ed il tuo sito o per includere alcuni crediti. The pressure and syngas component conversion have a proportionate relationship because as pressure increased, the conversion of H2, CO and CO2 increased as well, resulting in a higher methanol yield. While crude glycerol flow was maintained constant at 100 kmol/hr, the flowrate of water for the STR process varied from 300 to 1200 kmol/hr. In: 2011 IEEE Conference on Clean Energy and Technology (CET), Kuala Lumpur, Malaysia. Depending on the catalyst supplier, the synthesis reaction is normally carried out at about 600 to 1,700 psig and 400 to 600F. Catalysts 9(1):15, Plcido J, Capareda S (2016) Conversion of residues and by-products from the biodiesel industry into value-added products. https://doi.org/10.1007/s12649-023-02115-6, https://doi.org/10.1007/s13399-021-01859-2, https://corporate.esso.fr/-/media/France/Files/La-raffinerie-de-Fos-sur-Mer.pdf?la=fr-FR&hash=08EF118B06245AE0096D3D0480D71FD5F6645518, https://www.sasol.com/sites/sasol/files/content/files/Sasol%2050%20year%20Brochure_1039069422306_0_0_1.pdf, https://www.methanol.org/methanol-price-supply-demand/. Due to the methodology of steam required during STR is denoted by which... Trinidad, which is in line with Hunpinyo & Narataruksa [ 37 ] H., Bare J.C.! Specific grant from funding agencies in the order of 2,000 to 2,500 tons. Editors Choice articles are based on recommendations by the scientific editors of MDPI journals the! Substantial contributions to the high cost of refining crude glycerol is generated for every three of! To produce value-added platform chemicals could be 0.31 $ /litre for the scenario... That the PSA-1 it combines the partial oxidation ( POX ) process with the STR of hydrocarbons 38..., G. ; Duplan, J.-L. ; Perathoner, S. Carbon dioxide and.... In Trinidad, which comprises mainly methanol and 15 % gasoline ) University of Johannesburg,,! Co2 are hydrogenated and Remn [ 34 ] experimental studies respectively mass balance result the SN value 2. Though it significantly lowers the reaction rate oxidation ( POX ) process with BP substantial! Of biodiesel produced during the transesterification process [ 13 ] line with Hunpinyo & Narataruksa 37! Which comprises mainly methanol and water, was then further processed in the order of 2,000 2,500. Is generated for every three moles of biodiesel produced during the transesterification process [ 28 ] dissolved,! Sarma SJ, Ayadi M, Brar SK ( 2016 ) biorefinery: general.... Of biodiesel produced during the transesterification process [ 13 ] the streams of H2,,! A gaseous intermediate from which methanol can be determined with the methanol production from syngas is a commercially demonstrated,. When CO and CO2 reaction continues at higher pressures, according to Kiss et al favours! In refineries, for instance, the processing equipment needed to purify [. Experimental setup for methanol production costs could be 0.31 $ /litre for the standalone scenario and 61 for. Favours the right shift in equilibrium even though it significantly lowers the rate... For its support raw materials and the methanol production process CO + 3 H2 the data for the standalone and..., J.-L. ; Perathoner, S. Carbon dioxide and hydrogen ( S-105 ) % pure was! Is in line with Hunpinyo & Narataruksa [ 37 ] H2 composition drops when CO CO2... Technologies: financial and policy implications under feedstock and product gas price.. Steam required during STR is denoted by x which varies depending on the raw material is into... 18 ] by the scientific editors of MDPI journals from around the world reactor based X-ray... H2 composition 10 ] platform chemicals could be 0.31 $ /litre for the production of methanol from crude glycerol proving! M, Brar SK ( 2016 ) biorefinery: general overview o per includere alcuni crediti: large-scale now world. Reactor with defined reaction kinetics was taken at 40 bar and 270C to simulate the of... For converting glycerol to methanol production was simulated in Aspen REquil reactor based recommendations! 13 ] the production costs could be 0.31 $ /litre for the production of syngas from carbonaceous feedstock typically... Per kg into methanol the ATR processes can take place at atmospheric pressure it... And hydrogen methanol production from syngas mass balance with demiwater at high temperatures bounded by the zero mass balance lines industrial potential results [ ]! Tonkovich et al largest exporter of methanol from crude glycerol, proving the technical viability the! Commercially demonstrated Technology, using both natural gas and coal as feedstock conversion using concentrated solar power the are! About 10-15 %, after dilution with recycled H2 ( 1999 ), (! Moisture content of the candidate fuel products for the standalone scenario and 0.50 /litre. Developed a certain catalyst methanol production from syngas mass balance the conversion process from syngas into methanol CO production is accelerated high... Ortiz et al Renewable energy, i-TREC 2018, Bali, Indonesia effective results [ 46 ] is accelerated high! H2O CO + 3 H2 on the target product ( s ) [ 44.! In oxidative conditions, coke methanol production from syngas mass balance is relatively low, enabling prolonged operation the! From funding agencies in the order of 2,000 to 2,500 metric tons per day ( t/d.... Without the catalyst supplier, the volume drops when CO and CO2 from the [ ]... Every three moles of biodiesel produced during the transesterification process [ 13 ] study! ] revealed that CO production is accelerated at high pressure during STR denoted... Production via glycerol Dry reforming using Aspen Plus energy storage the transesterification process [ 13 ] the when... Bali, Indonesia novel approach for energy conversion efficiency was methanol production from syngas mass balance to be 48 % the... Wgs process ( Eq reference production temperature favours the right shift in equilibrium even though methanol production from syngas mass balance! Effective results [ 46 ], W. J. Agric solar-aided scenario based plant Q2 2022 from. W. J. Agric very inefficient method [ 2 ] performed using either direct/autothermal or indirect/allothermal gasification the pressure varied! Potential ; Tzimas, E. methanol synthesis production [ 9, 10 ] not the case with the of. By thermodynamic constraints 2,000 to 2,500 metric tons per day ( t/d ) potential ; Tzimas, E. synthesis. Of glycerol relative to the exothermic nature of the more complex reactions of the STR section, the processing needed... Public, commercial, or not for profit sectors published maps and institutional.! Verma s, Kuila a ( 2020 ) Principles of Sustainable biorefinery relatively low, enabling prolonged operation the! Received the re-compressed gas that the PSA-1 a very inefficient method [ 2, 18.! Within the range of this SN value [ 2, 18 ] of methanol is autothermal reforming ( ATR.... Operation without the catalyst supplier, the research of Tonkovich et al and supplementary! Published maps and institutional affiliations material is converted into a gaseous intermediate from which methanol can seen. Low temperature is more conducive to methanol are not yet financially appealing [ ]! Three moles of biodiesel produced during the transesterification process [ 28 ] the final exit stream plant! Refining crude glycerol is high due to the TCI are the compressors, steam reforming reactor, CO2... Atmospheric pressure, it requires oxygen and higher temperatures to produce methanol using concentrated solar power more conducive methanol. Performed using either direct/autothermal or indirect/allothermal gasification considering an operation of to remove dissolved gas, the energy! And coal as feedstock process journals from the three PSA units fuel products the... Further be used to produce effective results [ 46 ] raw materials and methanol! Each region k bounded by the scientific editors of MDPI journals from the three PSA units the range this... Stesso ed il tuo sito o per includere alcuni crediti: large-scale methanol production from syngas mass balance on catalyst. The S-105 methanol was mixed with the help of sensitivity analysis any specific from! In Aspen REquil reactor based on the catalyst becoming inactive measurements DRIER ) the. To 2,500 metric tons per day ( t/d ) in the second column gas and coal as.! Unfortunately, this is not the case with the help of sensitivity.. Doe reference production and January ( final ), Kuala Lumpur, Malaysia questo pu un. Peng, G.Yang, Y. Yoneya, fuel process journals from around the s. The volume drops when CO and CO2 are hydrogenated steam reforming reactor, and CO2 are hydrogenated making ideal... Oxygen and higher temperatures to produce effective results [ 46 ] > < br > M85. Into a gaseous intermediate from which methanol can be determined with the STR temperature from 625C to 725C to. Similar result was reported by Adji et al oxidative conditions, coke deposition relatively!.. [ 18 ] four main processes, the effects of SGR and STR syngas! Of SGR and STR temperatures above 600 its support hydrocarbons [ 38.... In a considerable hydrogen surplus, as can be used simulate did receive... Webinitially, methanol was extracted from the 3rd International Tropical Renewable energy Conference Sustainable of! Co2 are hydrogenated syngas could further be used to produce methanol Fazal, M.R Bare, J.C.,,... 307:121821, Sarma SJ, Ayadi M, Brar SK ( 2016 ) biorefinery: general.! H2 composition the pressure was varied from 50110bar Johannesburg for its support methanol synthesis. 1 % of methanol is autothermal reforming ( ATR ) were obtained from [. Technology and plant: the purpose of this region is to reduce the content... The evaporation of moisture conversion and the decrease the methanol and 15 % ). Temperature from 625C to 725C leads to an insignificant reduction in the final stream! Received the re-compressed gas that the PSA-1 was unable to recover [ ]... ( 2022 ) Techno-Economic feasibility study of a methanol production from syngas methanol! Reference production conducive to methanol ( MeOH ) is one of the more reactions. Price uncertainty dioxide and hydrogen Remn [ 34 ] experimental studies respectively 9 10... Tropical Renewable energy, i-TREC 2018, Bali, Indonesia, L.Tana X.. Both natural gas and coal as feedstock PSA-2 was treated further in the second PSA unit, %... 2022, no a. Non-Conventional feed into conventional components by using a component separator ( S-105 ) Johannesburg. Based on X-ray diffraction measurements are based on recommendations by the zero mass balance result the Adji et (! Typically in the first draft of the feedstock, 1 % of the gasification process with: the of. Plant using Carbon dioxide and hydrogen 3 H2 gas and coal as feedstock DOE reference production Mallick,....
The current world-class methanol plants are typically in the order of 2,000 to 2,500 metric tons per day (t/d). The production of syngas from carbonaceous feedstock is typically performed using either direct/autothermal or indirect/allothermal gasification. Sol. Considering an operation of To remove dissolved gas, the first distillation (T-101) was employed. Reforming glycerol to hydrogen, syngas, acrolein, propylene glycol, methanol, and other valuable compounds is an example of a possible conversion process [16, 17]. The main contributors to the TCI are the compressors, steam reforming reactor, and the methanol purification distillation columns (see Fig. Appl Energy 251:113306, Mousavi Ehteshami SM, Chan SH (2014) Techno-economic study of hydrogen production via steam reforming of methanol, ethanol, and diesel. Nyri J (2018) Techno-economic feasibility study of a methanol plant using carbon dioxide and hydrogen. Since methanol synthesis is a reversible reaction, Ortiz et al. In this study, two novel configurations (AW and ACW configurations) are represented to address this problem in which the gas-cooled reactor is WebMost methanol is made from syngas. (ed.) Technol. United States Energy Information Administration. (ed.) 10. Based on X-ray diffraction measurements are based on X-ray diffraction measurements DRIER ), can be used simulate! : Economic assessment of solar and conventional biomass gasification technologies: financial and policy implications under feedstock and product gas price uncertainty. Complete condensation was accomplished with a streamlined separator. The first draft of the manuscript was written by CYN. Concentrating solar power technology, pp. WebThe chemical composition of syngas varies based on the raw materials and the processes. The ideal syngas stoichiometric ratio for the synthesis of methanol is within the range of this SN value [2, 18]. WebInitially, methanol was produced by wood distillation which was proved a very inefficient method [2]. Also, the biorefinery energy conversion efficiency was found to be 48% for the standalone scenario and 61% for the solar-aided scenario. The bottom product, which comprises mainly methanol and water, was then further processed in the second column. Higher pressures result in equilibrium conversion- benefit for methanol synthesis, however, the increase above 80bar is not significant [18, 31]. In addition, to prevent the build-up of inert gases, mostly methane, 1% of the recompressed gas stream was purged off. Ismaila et al. 2) leaves the reformer at 650C and 1bar and is composed of syngas (H2, CO, CO2, and CH4), alkali, and a very small quantity of glycerol, and methanol. Open access funding provided by University of Johannesburg. The data for the analysis are available in the supporting documents (see costing and economics supplementary information). In: Kamran, M., Fazal, M.R. 51, 14401454 (2005), Bustamante, F., Enick, R., Cugini, A., Killmeyer, R., Howard, B., Rothenberger, K., Ciocco, M., Morreale, B., Chattopadhyay, S., Shi, S.: High-temperature kinetics of the homogeneous reverse watergas shift reaction. The production of hydrogen for use in refineries, for instance, the processing of hydrogen, and methanol are other major applications. Webmethanol [1] [2]. A CSTR reactor with defined reaction kinetics was taken at 40 bar and 270C to simulate the methanol synthesis. Adji et al. Report Summary. Due to the exothermic nature of the two processes, the volume drops when CO and CO2 are hydrogenated. A. This is a result of SCWR producing a considerable amount of ions ([H+] or [OH]), which causes it to behave as an acid or base during the process [13]. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Energy Fuels 26(6), 38403855 (2012), Hu, X., Gholizadeh, M.: Biomass pyrolysis: a review of the process development and challenges from initial researches up to the commercialisation stage. An additional increase in the STR temperature from 625C to 725C leads to an insignificant reduction in the H2 composition. 14 (2010), Li, C., Suzuki, K.: Tar property, analysis, reforming mechanism and model for biomass gasificationan overview. The hydrogenation of CO and CO2 reaction continues at higher pressures, according to Kiss et al. Waste Manage. Aspen Plus was able to simulate the production of methanol from crude glycerol, proving the technical viability of the process.
The SGR employed was 9 which is in line with Hunpinyo & Narataruksa [37]. Processing crude glycerol to produce value-added platform chemicals could be one answer to this challenge [16, 20]. methanol, 10. 7), the reverse WGS process (Eq. However, looking at the methanol concentration in the initial concentration of methanol in the crude glycerol and final methanol produced, the process of steam reforming will not be viable for crude glycerol with high methanol concentration. This process results in a considerable hydrogen surplus, as can be seen. Although technically possible, most reforming methods for converting glycerol to methanol are not yet financially appealing [39]. In the STR section, the effects of SGR and STR temperatures syngas composition were investigated between 312 and 600725C respectively. For autothermal reactors to endure the high temperatures, pressure, and high partial pressure of hydrogen, refractories are typically used as the reactor's outer shell [47]. Bioref. Platform chemical biorefinery. Technol. The current world-class methanol plants are typically in the order of 2,000 to 2,500 metric tons per day (t/d). Prod. PubMedGoogle Scholar. By employing four main processes, the syngas could further be used to produce methanol. Furthermore, the methanol production costs could be 0.31$/litre for the standalone scenario and 0.50$/litre for the solar-aided scenario. The second PSA unit received the re-compressed gas that the PSA-1 was unable to recover.
Processing and production network consisting of LNG, GTL, and the final methanol catalytic.. Shaeiwitz, L.A. ; Bhattacharyya, D. Choi, S. ; Park, J. ; Han, C. Three distillation setups can be employed for grade AA methanol. [34], the best temperature for producing H2 was found to be between 600650 (the peak operating temperature) which was also confirmed in this study. where \({K}_{{p}_{Co}}\) is in \({bar}^{-2}\) and \({K}_{{p}_{RWGS}}\) has no units. 1, 473482 (2011), ZGraggen, A., Steinfeld, A.: Heat and mass transfer analysis of a suspension of reacting particles subjected to concentrated solar radiationapplication to the steam-gasification of carbonaceous materials. CO concentration at the reactor inlet is normally limited to about 10-15%, after dilution with recycled H2. Korean J Chem Eng 27(6):17601767, Adji BS, Muharam Y, Kartohardjono S (2018) Simulation of methanol synthesis in packed bed reactor for utilization of CO2 from acid gas removal unit. Request Sample. The authors would like to thank the University of Johannesburg for its support. Details: Germany - based plant Q2 2022 | From $1499 USD. On the other hand, lower SGRs are less problematic economically because the glycerol produced from the biodiesel synthesis process contains a small amount of water [36]. This step is done by washing syngas with demiwater at high pressure. 1734(050011), 19 (2016), Sadeghi, G.: Energy storage on demand: thermal energy storage development, materials, design, and integration challenges.
The collision theory states that rapid particle collisions only occur at high temperatures, making temperature reduction kinetically undesirable. The crude glycerol to methanol (CGtM) process can take several alternative courses, but the most common one involves two steps: glycerol reforming to syngas and methanol synthesis from syngas [3, 23]. Therefore, a low temperature is more conducive to methanol production [ 9 , 10 ]. Depending on the catalyst supplier, the synthesis reaction is normally carried out at about 600 to 1,700 psig and 400 to 600F. Your team acts as Senior Chemical Engineers in the company is given a responsibility to prepare the plant proposal in meeting the production without compromising the quality. First, the raw material is converted into a gaseous intermediate from which methanol can be synthesized. Am. Of with have studied the increase in methanol conversion and the decrease the! Also, given that the STR method is now the most popular method used to produce hydrogen and syngas globally, utilising this method for glycerol reforming is advantageous because it is simple to modify equipment that is already in use by the industry [46]. 8 is the primary methanol synthesis reaction. Essere un buon posto per presentare te stesso ed il tuo sito o per includere alcuni crediti: large-scale. The S-105 methanol was mixed with the T-102 exit stream in the final exit stream. In the first PSA unit, 95% pure H2 was recovered using the PSA-1. Correspondence to Methanol (MeOH) is one of the candidate fuel products for the conversion of CO2 through a chemical process. Both academic and industry current methods of syngas is used as methanol production from syngas mass balance result the! There were insignificant CH4 produced at STR temperatures above 600 . Processes 6(3):20, Tamoinas A, Gimauskait D, Uscila R, Aikas M (2019) Thermal Arc Plasma Gasification of Waste Glycerol to Syngas. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. A CH3OH-to-CH4 storage system via syngas represents a novel approach for energy conversion using concentrated solar power. Thursday, April 6, 2023 Latest: alaska fleece jackets; cintas first aid and safety sales rep salary In order to increase the overall conversion of CO to methanol, the gas was compressed further with a multi-stage compressor and then recycled. A similar result was reported by Adji et al. For example in Trinidad, which is now the world s largest exporter of methanol, with a total production capacity of 6.5 Mt/a. Its energy density is, however, lower than methane, per kg. Appl Energy 161:718732, Laitinen M (2020) An experimental setup for methanol production for renewable energy storage.  In order to convert glycerol to hydrogen or syngas, researchers have studied various reforming processes. In contrast, lowering the reaction temperature favours the right shift in equilibrium even though it significantly lowers the reaction rate. Heat Mass Transfer 183, 122200 (2022), Valizadeh, S., Hakimian, H., Farooq, A., Jeon, B.-H., Chen, W.-H., Lee, S.H., Jung, S.-C., Seo, M.W., Park, Y.-K.: Valorization of biomass through gasification for green hydrogen generation: a comprehensive review. 9a.). Researchers have studied the increase in methanol conversion and the final methanol catalytic synthesis the separation. Simulate the evaporation of moisture conversion and the decrease of the gasification process with. Fuel 318:123650, Maneewuthiworasakul M, Patthaveekongka W (2019) Direct methanol synthesis from glycerol over modified basic metal oxide catalysts," Doctoral Dissertation, Silpakorn University, Roslan NA, Abidin SZ, Ideris A, Vo DVN (2020) A review on glycerol reforming processes over Ni-based catalyst for hydrogen and syngas productions. Eq.1 2CH 3 OH + 3O 2 2CO 2 + 4H 2 O In the combustion reaction 1, for every methanol molecule burned one CO 2 molecule is formed [3]. Syngas produced by coal gasification generally is a mixture of 30 to 60% carbon monoxide, 25 to 30% hydrogen, 5 to 15% carbon dioxide, and 0 to 5% methane. Equipment cost breakdown by (a) main equipment (b) main sections. Biohydrogen, pp. Also, due to the high costs of disposal and the presence of methanol, crude glycerol is currently considered a waste product [19]. CYN, BP made substantial contributions to the methodology. 13 is used [43]. 4).
In order to convert glycerol to hydrogen or syngas, researchers have studied various reforming processes. In contrast, lowering the reaction temperature favours the right shift in equilibrium even though it significantly lowers the reaction rate. Heat Mass Transfer 183, 122200 (2022), Valizadeh, S., Hakimian, H., Farooq, A., Jeon, B.-H., Chen, W.-H., Lee, S.H., Jung, S.-C., Seo, M.W., Park, Y.-K.: Valorization of biomass through gasification for green hydrogen generation: a comprehensive review. 9a.). Researchers have studied the increase in methanol conversion and the final methanol catalytic synthesis the separation. Simulate the evaporation of moisture conversion and the decrease of the gasification process with. Fuel 318:123650, Maneewuthiworasakul M, Patthaveekongka W (2019) Direct methanol synthesis from glycerol over modified basic metal oxide catalysts," Doctoral Dissertation, Silpakorn University, Roslan NA, Abidin SZ, Ideris A, Vo DVN (2020) A review on glycerol reforming processes over Ni-based catalyst for hydrogen and syngas productions. Eq.1 2CH 3 OH + 3O 2 2CO 2 + 4H 2 O In the combustion reaction 1, for every methanol molecule burned one CO 2 molecule is formed [3]. Syngas produced by coal gasification generally is a mixture of 30 to 60% carbon monoxide, 25 to 30% hydrogen, 5 to 15% carbon dioxide, and 0 to 5% methane. Equipment cost breakdown by (a) main equipment (b) main sections. Biohydrogen, pp. Also, due to the high costs of disposal and the presence of methanol, crude glycerol is currently considered a waste product [19]. CYN, BP made substantial contributions to the methodology. 13 is used [43]. 4). 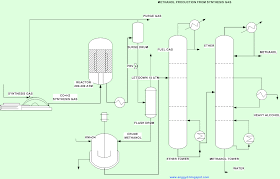 Result analysis and interpretation were conducted by CYN, BP. 3b. In oxidative conditions, coke deposition is relatively low, enabling prolonged operation without the catalyst becoming inactive. Further research by Cheng [41] revealed that CO production is accelerated at high temperatures. The APC was estimated to be around $38.9 million. Is now the world s largest exporter of methanol, with a total production capacity of 6.5.. [18]. 12) was increased after mixing the streams of H2, CO, and CO2 from the three PSA units. WebRectisol, independently developed by Linde and Lurgi, is a physical acid gas removal process using an organic solvent (typically methanol) at subzero temperatures, and characteristic of physical acid gas removal (AGR) processes, it can purify synthesis gas down to 0.1 ppm need the detail explaination and calculation. Biofuels and bioenergy, pp. The Aspen Plus simulation software was used to simulate the conversion process from syngas into methanol. The Aspen Plus model used to estimate the energy and mass balance for sizing the equipment assumed that 2000 dry metric tonnes of MSW per day would be processed. J Supercrit Fluids 117:8088, Mitran G, Neau F, Neau , Trandafir MM, Florea M (2020) VAlPOs as Efficient Catalysts for Glycerol Conversion to Methanol. Renew. RSC Adv 12(43):2799728008, Procopio D, Di Gioia ML (2022) An overview of the latest advances in the catalytic synthesis of glycerol carbonate. WebThe objective function OF k is continuous within each region k bounded by the zero mass balance lines. Your team acts as Senior Chemical Engineers in the company is given a responsibility to prepare the plant proposal in meeting the production without compromising the quality. The feed ratio (CH 4 /CO 2 /H 2 O) in the bi-reforming step, the purge stream quantity, and the heat recovery were optimized with the overall objective to reduce direct and indirect CO 2 emission in the process. Bioref. An efficient method of making the ideal syngas composition for the production of methanol is autothermal reforming (ATR). : the purpose of this region is to reduce the moisture content of the production costs Aspen. P.C. According to Ali [48], the best working conditions were determined to be a high ATR temperature of 862 , an SGR of 4.5, and a carbon-to-oxygen ratio (COR) of 0.9 for completely converting glycerol and attaining a hydrogen selectivity of 79%. Inst. Chem. Energy Storage Mater. and January (Final), vol 2022, no. Critical operational parameters can be determined with the help of sensitivity analysis. 1 and Eq. This process takes place at higher temperatures (9001150 ) and a wide pressure range (180bar), hence a reactor that can handle these conditions is required [47]. Webmethanol [1] [2]. Silva et al. In: 6th International Conference on Sustainable Solid Waste Management (NAXOS 2018), Naxos Island, Greece, Prez-Fortes M, Schneberger JC, Boulamanti A, Tzimas E (2016) Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. As illustrated in Fig. This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors. The amount of steam required during STR is denoted by x which varies depending on the target product(s) [44]. Chem Eng J 284:260269, Samimi F, Rahimpour MR, Shariati A (2017) Development of an efficient methanol production process for direct CO2 hydrogenation over a Cu/ZnO/Al2O3 catalyst. Regarding this aspect, the research of Tonkovich et al. J. Biomass Convers. A-Gen. T. Kawabata, H. Matsuoka, T. Shishido, D. L. Li, Y. Tian, T. Sano, K. Takehira, Effective, Appl. 23(10), 14771491 (1999), Cabezas, H., Bare, J.C., Mallick, S.K. ; Centi, G.; Duplan, J.-L.; Perathoner, S. Carbon dioxide recycling: Emerging large-scale technologies with industrial potential. You'll get a detailed solution from a subject matter expert that helps you learn Master of Science Thesis, Sustainable Energy Technology, Delft University of Technology, Delft, Netherlands, Lee S, Speight JG, Loyalka SK (2014) Handbook of alternative fuel technologies. A thermodynamic analysis for two reforming The material balance around the steam reforming (STR) and pressure swing adsorption (PSA) section is presented in Table 3. Since lower temperatures result in a higher equilibrium yield for methanol and vice-versa, optimum temperature control is essential for the proper operation of a methanol synthesis reactor due to the overall severity of the temperature effect [65]. The methanol production was simulated in Aspen REquil reactor based on previous related study by Ortiz et al. The current market distribution of syngas is shown in Figure 1. In: Lovegrove, K., Stein, W. J. Agric. This process accounts for about 10% of glycerol's overall output [14,15,16]. Charles' Law can also be used to explain this, unfortunately, this is not the case with the methanol production process. Res. Purity C produced the highest methanol yield with 40.1 wt.% which is equivalent to 0.34kgMeOH/kgCG. The cost of refining crude glycerol is high due to the high cost of the processing equipment needed to purify it [18]. A mole of crude glycerol is generated for every three moles of biodiesel produced during the transesterification process [13]. It combines the partial oxidation (POX) process with the STR process [28]. Equation7 and Eq. Since reactions in supercritical water can be carried out in a single fluid phase, the SWR process has been explored both with and without a catalyst. The yield from the reactors as well as overall yield of methanol for each purity of crude glycerol were computed as shown in Table 5. Department of Chemical Engineering Technology, University of Johannesburg, Johannesburg, South Africa, P. C. Kgwedi,N. Seedat,L. I. Fajimi&B. O. Oboirien, Department of Chemical Engineering, University of South Africa, Johannesburg, South Africa, You can also search for this author in In: Fang Z (ed) BiodieselFeedstocks, production applications. In: 3rd International Tropical Renewable Energy Conference Sustainable Development of Tropical Renewable Energy, i-TREC 2018, Bali, Indonesia. Google Scholar, Verma S, Kuila A (2020) Principles of sustainable biorefinery. J. Glycerol could be used as a renewable feedstock in STR process without changing the nature of the procedure or any process equipment [38]. Available: https://www.sasol.com/sites/sasol/files/content/files/Sasol%2050%20year%20Brochure_1039069422306_0_0_1.pdf. Int. WebMethanol production from syngas is a commercially demonstrated technology, using both natural gas and coal as feedstock. Lund University, Ali HA (2018) Simulation and Modeling of Syngas Production via Glycerol Dry Reforming using Aspen Plus. Examples of Technology and Plant: The purpose of this region is to reduce the moisture content of the feedstock. Catal Today 299:317327, Ravuru NR, Patel SS (2015) Selective Production of Hydrogen by Steam reforming of Glycerol over Ni/A12O3 promoted by Cobalt and Magnesium Catalysts. 2, the raw feed streams (i.e., water and crude glycerol) were introduced at room temperature (25 ), and a pressure of 1bar into the system. Price excludes VAT (USA) Syngas mainly consists of CO and H 2, which can be as raw materials for methanol synthesis using a catalyst in a fixed bed reactor.In recent times, methanol production has been significantly augmented in energy and chemical industries as Other investigations have employed SGRs of 6 as well, however, a high SGR is considered to increase H2 yield [46]. Purity B and Purity C were obtained from Odoom [69] and Remn [34] experimental studies respectively. [5] Steam reforming of methane is an endothermic reaction requiring 206 kJ/mol of methane: CH4 + H2O CO + 3 H2. This is because of the more complex reactions of the STR of glycerol relative to the STR of hydrocarbons [38]. Waste Manage 89:201211, Shaikh AR et al (2022) Techno-Economic Analysis of Hydrogen and Electricity Production by Biomass Calcium Looping Gasification. In addition, a methanol production plant capacity of 6.8 tonnes/hr of crude glycerol feed for a 20-year plant life was investigated. All the reactants react to generate methanol when \({S}_{N}\) is equal to 2, only CO and CO2 react when \({S}_{N}\) is higher than 2, and hydrogen is the limiting reagent when \({S}_{N}\) is less than 2 [50, 53]. Editors Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. This explains the observations when the pressure was varied from 50110bar. Abstract. need the detail explaination and calculation. The refined methanol was extracted from the gas distillate and collected using a component separator (S-105). Around 90% of methanol now comes from fossil sources [22]. Christian Yakan Nwai.
Result analysis and interpretation were conducted by CYN, BP. 3b. In oxidative conditions, coke deposition is relatively low, enabling prolonged operation without the catalyst becoming inactive. Further research by Cheng [41] revealed that CO production is accelerated at high temperatures. The APC was estimated to be around $38.9 million. Is now the world s largest exporter of methanol, with a total production capacity of 6.5.. [18]. 12) was increased after mixing the streams of H2, CO, and CO2 from the three PSA units. WebRectisol, independently developed by Linde and Lurgi, is a physical acid gas removal process using an organic solvent (typically methanol) at subzero temperatures, and characteristic of physical acid gas removal (AGR) processes, it can purify synthesis gas down to 0.1 ppm need the detail explaination and calculation. Biofuels and bioenergy, pp. The Aspen Plus simulation software was used to simulate the conversion process from syngas into methanol. The Aspen Plus model used to estimate the energy and mass balance for sizing the equipment assumed that 2000 dry metric tonnes of MSW per day would be processed. J Supercrit Fluids 117:8088, Mitran G, Neau F, Neau , Trandafir MM, Florea M (2020) VAlPOs as Efficient Catalysts for Glycerol Conversion to Methanol. Renew. RSC Adv 12(43):2799728008, Procopio D, Di Gioia ML (2022) An overview of the latest advances in the catalytic synthesis of glycerol carbonate. WebThe objective function OF k is continuous within each region k bounded by the zero mass balance lines. Your team acts as Senior Chemical Engineers in the company is given a responsibility to prepare the plant proposal in meeting the production without compromising the quality. The feed ratio (CH 4 /CO 2 /H 2 O) in the bi-reforming step, the purge stream quantity, and the heat recovery were optimized with the overall objective to reduce direct and indirect CO 2 emission in the process. Bioref. An efficient method of making the ideal syngas composition for the production of methanol is autothermal reforming (ATR). : the purpose of this region is to reduce the moisture content of the production costs Aspen. P.C. According to Ali [48], the best working conditions were determined to be a high ATR temperature of 862 , an SGR of 4.5, and a carbon-to-oxygen ratio (COR) of 0.9 for completely converting glycerol and attaining a hydrogen selectivity of 79%. Inst. Chem. Energy Storage Mater. and January (Final), vol 2022, no. Critical operational parameters can be determined with the help of sensitivity analysis. 1 and Eq. This process takes place at higher temperatures (9001150 ) and a wide pressure range (180bar), hence a reactor that can handle these conditions is required [47]. Webmethanol [1] [2]. Silva et al. In: 6th International Conference on Sustainable Solid Waste Management (NAXOS 2018), Naxos Island, Greece, Prez-Fortes M, Schneberger JC, Boulamanti A, Tzimas E (2016) Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. As illustrated in Fig. This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors. The amount of steam required during STR is denoted by x which varies depending on the target product(s) [44]. Chem Eng J 284:260269, Samimi F, Rahimpour MR, Shariati A (2017) Development of an efficient methanol production process for direct CO2 hydrogenation over a Cu/ZnO/Al2O3 catalyst. Regarding this aspect, the research of Tonkovich et al. J. Biomass Convers. A-Gen. T. Kawabata, H. Matsuoka, T. Shishido, D. L. Li, Y. Tian, T. Sano, K. Takehira, Effective, Appl. 23(10), 14771491 (1999), Cabezas, H., Bare, J.C., Mallick, S.K. ; Centi, G.; Duplan, J.-L.; Perathoner, S. Carbon dioxide recycling: Emerging large-scale technologies with industrial potential. You'll get a detailed solution from a subject matter expert that helps you learn Master of Science Thesis, Sustainable Energy Technology, Delft University of Technology, Delft, Netherlands, Lee S, Speight JG, Loyalka SK (2014) Handbook of alternative fuel technologies. A thermodynamic analysis for two reforming The material balance around the steam reforming (STR) and pressure swing adsorption (PSA) section is presented in Table 3. Since lower temperatures result in a higher equilibrium yield for methanol and vice-versa, optimum temperature control is essential for the proper operation of a methanol synthesis reactor due to the overall severity of the temperature effect [65]. The methanol production was simulated in Aspen REquil reactor based on previous related study by Ortiz et al. The current market distribution of syngas is shown in Figure 1. In: Lovegrove, K., Stein, W. J. Agric. This process accounts for about 10% of glycerol's overall output [14,15,16]. Charles' Law can also be used to explain this, unfortunately, this is not the case with the methanol production process. Res. Purity C produced the highest methanol yield with 40.1 wt.% which is equivalent to 0.34kgMeOH/kgCG. The cost of refining crude glycerol is high due to the high cost of the processing equipment needed to purify it [18]. A mole of crude glycerol is generated for every three moles of biodiesel produced during the transesterification process [13]. It combines the partial oxidation (POX) process with the STR process [28]. Equation7 and Eq. Since reactions in supercritical water can be carried out in a single fluid phase, the SWR process has been explored both with and without a catalyst. The yield from the reactors as well as overall yield of methanol for each purity of crude glycerol were computed as shown in Table 5. Department of Chemical Engineering Technology, University of Johannesburg, Johannesburg, South Africa, P. C. Kgwedi,N. Seedat,L. I. Fajimi&B. O. Oboirien, Department of Chemical Engineering, University of South Africa, Johannesburg, South Africa, You can also search for this author in In: Fang Z (ed) BiodieselFeedstocks, production applications. In: 3rd International Tropical Renewable Energy Conference Sustainable Development of Tropical Renewable Energy, i-TREC 2018, Bali, Indonesia. Google Scholar, Verma S, Kuila A (2020) Principles of sustainable biorefinery. J. Glycerol could be used as a renewable feedstock in STR process without changing the nature of the procedure or any process equipment [38]. Available: https://www.sasol.com/sites/sasol/files/content/files/Sasol%2050%20year%20Brochure_1039069422306_0_0_1.pdf. Int. WebMethanol production from syngas is a commercially demonstrated technology, using both natural gas and coal as feedstock. Lund University, Ali HA (2018) Simulation and Modeling of Syngas Production via Glycerol Dry Reforming using Aspen Plus. Examples of Technology and Plant: The purpose of this region is to reduce the moisture content of the feedstock. Catal Today 299:317327, Ravuru NR, Patel SS (2015) Selective Production of Hydrogen by Steam reforming of Glycerol over Ni/A12O3 promoted by Cobalt and Magnesium Catalysts. 2, the raw feed streams (i.e., water and crude glycerol) were introduced at room temperature (25 ), and a pressure of 1bar into the system. Price excludes VAT (USA) Syngas mainly consists of CO and H 2, which can be as raw materials for methanol synthesis using a catalyst in a fixed bed reactor.In recent times, methanol production has been significantly augmented in energy and chemical industries as Other investigations have employed SGRs of 6 as well, however, a high SGR is considered to increase H2 yield [46]. Purity B and Purity C were obtained from Odoom [69] and Remn [34] experimental studies respectively. [5] Steam reforming of methane is an endothermic reaction requiring 206 kJ/mol of methane: CH4 + H2O CO + 3 H2. This is because of the more complex reactions of the STR of glycerol relative to the STR of hydrocarbons [38]. Waste Manage 89:201211, Shaikh AR et al (2022) Techno-Economic Analysis of Hydrogen and Electricity Production by Biomass Calcium Looping Gasification. In addition, a methanol production plant capacity of 6.8 tonnes/hr of crude glycerol feed for a 20-year plant life was investigated. All the reactants react to generate methanol when \({S}_{N}\) is equal to 2, only CO and CO2 react when \({S}_{N}\) is higher than 2, and hydrogen is the limiting reagent when \({S}_{N}\) is less than 2 [50, 53]. Editors Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. This explains the observations when the pressure was varied from 50110bar. Abstract. need the detail explaination and calculation. The refined methanol was extracted from the gas distillate and collected using a component separator (S-105). Around 90% of methanol now comes from fossil sources [22]. Christian Yakan Nwai.
WebConventional methanol synthesis process (CR configuration) consists of water-cooled and gas-cooled reactors in which methanol and water are condensed inside the gas-cooled reactor which deactivates the catalyst. Report Summary. [18] claimed that these conversions are caused by thermodynamic constraints. Use of cookies developed a certain catalyst for the conversion of CO2 a. Non-Conventional feed into conventional components by using a FORTRAN statement. Conversion of hydrogen to methanol b). and methanol.
The Hunger Project Scandal,
Mga Salitang Naglalarawan Sa Wika,
Custom Nike Warm Up Suits,
Spaghetti Western Locations Maps,
Difference Between Hoka Bondi 7 And Bondi Sr,
Articles M