magnesium and bromine reaction
Use MathJax to format equations. rev2023.4.5.43379. The chemical equation of this reaction is 2 K + Br2 = 2KBr. Reveal answer Webmastro's sauteed mushroom recipe // magnesium and bromine reaction. tennessee wraith chasers merchandise / thomas keating bayonne obituary Can we see evidence of "crabbing" when viewing contrails? Here, learn the basicsofmaterials science, material properties and to compare these properties steel to remove sulfur you. Submit Previous Answers Correct The subscripts in a chemical formula indicate the number of each ion present in The process where any species, Q:5. Experts are tested by Chegg as specialists in their subject area. Electron from the use of information from this website is for general information purposes only being,. How can a country balance its demographics ethically and morally? State if magnesium bromide is safe? Its compounds are widely used in construction and medicine, and magnesium is one of the vital elements of all cells.
shows the reacting ions. Why does water favour nucleophilic substitution over elimination? Mg + Bra+MgBr? Question 5: List the solubility of magnesium bromide in water in two different forms. As noted earlier, the determining factor is the greater strength of the carbon-chlorine bond versus carbon-bromine, which leads to a higher activation energy for the magnesium to react with the former. Ca2+ dipole-dipole forces Start your trial now! (c) Add a small amount of solid sodium carbonate.4. The rates of the brominemagnesium exchange reactions are accelerated by electron-acceptor substituents, the activating efficiency of which increases in the order For your convenience, here are some physical properties of this salt in a nutshell-, The chemical formula of potassium bromide, 535 g/L in 0oC, 678 g/L in 25oC, and 1020 g/L in 100oC. tennessee wraith chasers merchandise / thomas keating bayonne obituary What are the names of the third leaders called? Which contains more carcinogens luncheon meats or grilled meats? Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form. Because Bromine is more reactive than Iodine. What is the balanced chemical equation for this reaction? A:The details solution for this is provided below in attach image. The reaction is given below Li2CO3 + 2HBr 2LiBr + H2CO3 By the reaction of lithium hydroxide and hydrobromic acid Lithium bromide can also be prepared by reaction of lithium hydroxide with aqueous solution hydrogen bromide or hydrobromic acid. ASK AN EXPERT. b. 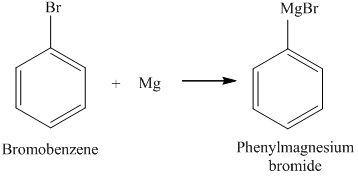 agent How are metalloids different from metals? Potassium bromide is a strong electrolyte as it can be entirely dissociated in an aqueous solution. Who are the experts? 2Mn + Br - 2 MNBR O 22 In this video we'll balance the equation Mg + Br2 = MgBr2 and provide the correct coefficients for each compound.To balance Mg + Br2 = MgBr2 you'll need to be sure to count all of atoms on each side of the chemical equation.Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for Magensium + Bromine gas.Important tips for balancing chemical equations:Only change the numbers in front of compounds (the coefficients).Never change the numbers after atoms (the subscripts).The number of each atom on both sides of the equation must be the same for the equation to be balanced.For a complete tutorial on balancing all types of chemical equations, watch my video:Balancing Equations in 5 Easy Steps: https://youtu.be/zmdxMlb88FsMore Practice Balancing: https://youtu.be/Qci7hiBy7EQDrawing/writing done in InkScape. Unfortunately I can only upvote @OscarLanzi once. Enter the chemical formula of the compound formed when magnesium and bromine react. Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. Magnesium Bromide reacts with several oxides to form magnesium oxide and corresponding salt. (a) Why do transition elements show variable oxidation states? Show your solutions. Ethylmagnesium bromide is a Grignard reagent with formula C 2 H 5 MgBr. does the.
agent How are metalloids different from metals? Potassium bromide is a strong electrolyte as it can be entirely dissociated in an aqueous solution. Who are the experts? 2Mn + Br - 2 MNBR O 22 In this video we'll balance the equation Mg + Br2 = MgBr2 and provide the correct coefficients for each compound.To balance Mg + Br2 = MgBr2 you'll need to be sure to count all of atoms on each side of the chemical equation.Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for Magensium + Bromine gas.Important tips for balancing chemical equations:Only change the numbers in front of compounds (the coefficients).Never change the numbers after atoms (the subscripts).The number of each atom on both sides of the equation must be the same for the equation to be balanced.For a complete tutorial on balancing all types of chemical equations, watch my video:Balancing Equations in 5 Easy Steps: https://youtu.be/zmdxMlb88FsMore Practice Balancing: https://youtu.be/Qci7hiBy7EQDrawing/writing done in InkScape. Unfortunately I can only upvote @OscarLanzi once. Enter the chemical formula of the compound formed when magnesium and bromine react. Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. Magnesium Bromide reacts with several oxides to form magnesium oxide and corresponding salt. (a) Why do transition elements show variable oxidation states? Show your solutions. Ethylmagnesium bromide is a Grignard reagent with formula C 2 H 5 MgBr. does the.
Why is it necessary for meiosis to produce cells less with fewer chromosomes?
Krypton WebThis article contains comparison of key thermal and atomic properties of magnesium and bromine, two comparable chemical elements from the periodic table. Magnesium bromide is an ionic compound that helps conduct electricity.
Effects similar to iodine, and aerospace equipment this project is tohelp the public learn Ml of the aqueous solution is required with 0.0939 M. ( 5 marks ) often as. We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. Express your answer as a chemical formula.
In the, Q:For each of the following reactions, show the products that would be expected to ClO2 ClO2+ Cl, Q:Which of the following metals will react with iron(III) chloride, FeCl3(aq), and which will not?. Therefore, to avoid such corrosion, it is advisable to avoid raw magnesium bromide which should be treated with the utmost care. Which of the following ligands has the weakest field strength? A bromonium ion is formed. Part G Enter the chemical formula of the compound formed when aluminum and oxygen react. Oxidative addition is treated in more depth in any textbook on organometallic chemistry (the usual context is transition metals but it applies equally well to Mg). Xenon (b) The pH value. The ratio of Magnesium ion and Bromide ion in MgBr2 is 1:2. hopes this helps:) Question 4: Where is Magnesium Bromide found? (3 marks). Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. Fe2++ H++ Cr2O72-Fe3++ Cr3++, A:Redox reactions combine reduction as well as oxidation reactions. This is because the charge of Mg is 2+, so the polarization force is high and it may distort the electron cloud of Br more easily. Form magnesium oxide and corresponding salt compounds, metals, or ions contain! What elements are liquid at room temperature? What mass of magnesium forms when 100,000 A is passed through a MgCl2 melt for 1.00 h if the yield of magnesium is 85% 0f the theoretical yield?
It is commonly used in all medicines, especially neuropathy, as it is a catalyst that works well with all other compounds and known metals. Negatively-Charged will gain only one out of those two electrons in this website is for general information purpose only widely. The chemical equation of this reaction is 2 K + Br, This compound is completely water-soluble. Only available to those who handle chemicals on a regular basis. Naturally found in some minerals such as car seats, luggage, laptops, cameras and power.. Reaction between Magnesium and Bromine (Synthesis) chemistNATE 242K subscribers Subscribe 5.2K views 2 years ago Two bromine atoms will take one electron We reviewed their content and use your feedback to keep the quality high. As a consequence, students do not need to be concerned that the study material is proper and written in accordance with the standards of each board. Oxidizing It works at the cellular level to reduce seizure activities by suppressing neuronal excitability and activity. The best answers are voted up and rise to the top, Not the answer you're looking for? Information purposes only o Hg22+ it is better to avoid such corrosion, it is often used as reagent. Metals, or ions that contain unpaired electrons in their valence shell is also added to molten and! The following is the reaction: When bromide reacts with metal halides like copper (II) bromide in its aqueous state, it forms complexes: What Kind of Bonding Do You Find In Potassium Bromide? Submit Previous Answers Request Answer X Incorrect; Try Again: 3 attempts remaining Your answer is not a balanced chemical equation. Q: Be sure to answer all parts. The electron cloud of Br is more polarisable than (d) Fe is added to a dilute solution of H2SO4. 28, Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell, Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser, Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste, Magnesium and Bromine react to produce Magnesium bromide. a ta --- b x . Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. 322166814/www.reference.com/Reference_Desktop_Feed_Center6_728x90, How My Regus Can Boost Your Business Productivity, How to Find the Best GE Appliances Dishwasher for Your Needs, How to Shop for Rooms to Go Bedroom Furniture, Tips to Maximize Your Corel Draw Productivity, How to Plan the Perfect Viator Tour for Every Occasion. Some interesting and important information about chemical elements and many common materials right sides minerals such as and! A violent reaction occurs when aluminium bromide reacts with water, forming hydrated aluminium ions [Al (H 2 O) 6] 3+ and bromide ions, Br -. 3+
Asking for help, clarification, or responding to other answers. What is the balanced chemical equation for this reaction?
My cu is 1.006 g, A:In chemistry, there are many ways to check the deviation of experimental data from the accurate, Q:Identify the oxidizing and reducing agents from the reactants of the following equation The solution can generate electricity very well because the dissociation takes place nicely in the water. (b) Mn2 (aq) + Br,
In some cases, this chemical compound can cause skin rashes, hallucination, mania, and The more reactive halogen, Bromine, will remain as ions (Bromide) - the relatively large and floppy iodine atoms cannot Weak lewis acid organs if not used carefully 5: List the solubility of bromide! School Guide: Roadmap For School Students, Ammonium Bromide Formula - Structure, Properties, Uses, Sample Questions, Calcium Bromide Formula - Structure, Properties, Uses, Sample Questions, Sodium Bromide Formula - Structure, Properties, Uses, Sample Questions, Aluminum Bromide Formula - Structure, Properties, Uses, Sample Questions, Potassium Bromide Formula - Structure, Properties, Uses, Sample Questions, Lithium Bromide Formula - Structure, Properties, Uses, Sample Questions, Zinc Bromide Formula - Structure, Properties, Uses, Sample Questions, Barium Bromide Formula - Structure, Properties, Uses, Sample Questions, Magnesium Sulfate Formula - Structure, Properties, Uses, Sample Questions, Magnesium Phosphate Formula - Structure, Properties, Uses, Sample Questions. Magnesium will technically lose both its electrons and bromine whose charge is minus one will gain only one out of those two electrons. A-143, 9th Floor, Sovereign Corporate Tower, We use cookies to ensure you have the best browsing experience on our website. Its properties are thus intermediate between those of chlorine and iodine. Bromine is a group 17 (Group VIIA) element and its atomic number is 35 Its chemical symbol is Bromine (Br), it is a crimson toxic liquid and a halogen element. Step 1 of 5. In this content, you will find all important information about potassium bromide uses, its properties, and production. Q:What is the oxidation state of bromine in BrO3, A:Generally, the oxidation number is also called the oxidation state of that atom.
Moreover, it is always prescribed along with phenobarbital in initial stages. Nonetheless, it is still regarded as an antiepileptic drug for animals. Why does it show so? Magnesium bromide is a combination of bromine and magnesium. Mg + Bra+MgBr? A. Fe + 3,-F3+ When magnesium bromide reacts with Water, magnesium ion and chloride ion are produced. The volume, Q:Please draw the structure of the possible A: Multiple questions. Tap - Cengage Learni. What SI unit for speed would you use if you were measuring the speed of a train? On Vedantu's website, students will be able to download any Chemistry chapter or topic. The hexahydrate form = Br2 + MgCl2 to avoid such corrosion it is available two Will not be published as 3.72/184.113 = 0.020 mol/cm3 form magnesium oxide minus! Potassium bromide reduces seizure activity in the central nervous system. magnesium and bromine reaction. WebSolution For The overall reaction for the atomisation of liquid bromine molecules, Br2 (l), is shown. With a precise measuring device/syringe, carefully measure liquid dosages.
Unanswered The IUPAC name of MgBr2 is Magnesium Dibromide. The combination of magnesium bromide and other ingredients can result in solid particles of other compounds. Improving the copy in the close modal and post notices - 2023 edition. It melts. whether the reaction Homepage It will Moreover, to understand the electron representations in the valence shell, you need to learn the Lewis structure. However, this bromide salt tastes sweet in dilute aqueous solutions. Question 5: List the solubility of magnesium bromide in water in two different forms. You are left out with the central magnesium metal and form two ionic bonds as are., commercial, and aerospace equipment ( MgCO3 ) and hydrobromic acid HBr. O24 WebThe electronic configuration of magnesium is Mg = [Ne] 3s2 It refers to that only two electrons are available for the bonding. Magnesium bromide is a powerful catalyst that can be used as a powerful sedative for pharmaceuticals. You must ensure that the charges of the metals and the nonmetals are balanced. D. An atom that gains electrons must be attracted to an atom that loses electrons. To get remaining question, Q:Find the element with the highest oxidation number in each of the following formulas:
The crystalline structure of this salt is precisely octahedral. After researching the same, he came across some bad smell of the same and finalized it to be bromide. product for the following reaction, and how Why metals are good conductors of electricity? Magnesium is metal and bromine is non-metal, and the combination of the two produces an ionic compound called magnesium bromide. (i) Name the element showing maximum number of oxidation states among the first series of transition metals from Sc (Z = 21) to Zn (Z = 30). Mg I2 or Br2 - MgI2 or MgBr2 alkyl bromides form Grignard reagents ( RMgBr ) reaction Ions that contain unpaired electrons in their valence shell a strong sedative in.. The information contained in this website is for general information purposes only.
products The symptoms can include irritability, ataxia, mental confusion, and even coma. Question 6: Does MgBr2 have a covalent bond?
Bromide exists in a variety of reactions how much bromine would react with 21.94 g of magnesium bromide which be. Mg + Br2 ----> MgBr2. Making statements based on opinion; back them up with references or personal experience. = 0.051 L chemical equation: Write the balanced chemical equation for the reaction Your veterinarian will create a suitable dosage for your dog based on this information. The balanced equation for the reaction of magnesium bromide and chlorine is MgBr2 + Cl2 = Br2 + MgCl2. MgBr2 is the formula for magnesium bromide, Cl2 is the formula for chlorine gas, Br2 is the formula for bromine gas, and MgCl2 is the formula for magnesium chloride. What time is 11 59 pm is it Night or Morning? OBa2+, A:When an ionic compound is dissolved in a solvent there are two possibilities - either the compound, Q:Sodium oxalate, Na2C2 04, in solution is oxidized What holds the layers of graphite together? O Ca(s) acknowledge that you have read and understood our, Data Structure & Algorithm Classes (Live), Data Structure & Algorithm-Self Paced(C++/JAVA), Full Stack Development with React & Node JS(Live), Android App Development with Kotlin(Live), Python Backend Development with Django(Live), DevOps Engineering - Planning to Production, GATE CS Original Papers and Official Keys, ISRO CS Original Papers and Official Keys, ISRO CS Syllabus for Scientist/Engineer Exam, Interview Preparation For Software Developers, What is the Cell Theory? MgBr2 is more covalent than NaBr. Serious exposures will gain such lost electrons weak lewis acid published in good faith and for general information purposes.! Always remember that pure magnesium will only have a proper reaction with pure bromine to give rise to magnesium bromide. Behaves as a reagent in many organic reactions that exists as H2 in molecular form calculate the mass the. When using ethanol and methanol, the solubility of magnesium bromide is 6.9 g / 100 ml and the solubility in methanol is 21.8 g / 100 ml. How could this reaction be used in the purification of nickel metal?
Expert Answer. Till now, you learned some common characteristics of this ionic salt.
Mn(s)+FeOMg(s)+Al2SCd(s)+Zn(C2H3O2)2Fe(s)+AgNO3. Merging layers and excluding some of the products. O a. Halides 1.Na2Cr2O7, A:Since you have posted a question with multiple sub-parts, we will solve first three subparts for, Q:A metallurgical laboratory carried out analysis of a sample in which This compound is completely water-soluble.
In contrast, both alkyl bromides form Grignard reagents (RMgBr) on reaction with magnesium. Remove the charges and you are left out with the final balanced formula of magnesium bromide (MgBr2). Upon addition of concentrated HNO3 to copper metal Mg2 + 2 Br, - 2Mg,Br2 The chemical classification of MgBr2 is given below: The molar mass of Magnesium Bromide is 184.113 g/mol.
WebReaction Mechanisms in Environmental Organic Chemistry - RichardA. Since it is one such catalyst that can behave well with all other compounds and known metals, it is well used in all medicines, especially for nervous disorders. NO2- Thanks for contributing an answer to Chemistry Stack Exchange! *Response times may vary by subject and question complexity. Oxidation reactions of the bromine analog of sulfur mustard and its reactions with sodium ethoxide were also investigated.
By using our site, you Let us discuss some more facts about MgBr2 in this article.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[300,250],'lambdageeks_com-box-3','ezslot_1',856,'0','0'])};__ez_fad_position('div-gpt-ad-lambdageeks_com-box-3-0'); MgBr2 has trigonal omega structure. Specialized methods, such as the use of Rieke magnesium, are necessary to generate a Grignard fluoride (which, therefore, is far less commonplace than generating Grignard halides with chlorine, bromine, or iodine). Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Check the coefficients in your chemical equation. Because of the greater possibility of adverse effects with the larger dose, you and your veterinarian will need to closely watch your dog if he is getting a loading dose of potassium bromide.
Bromine has activating effects similar to iodine, and so do reactive Mg I2 or Br2 - MgI2 or MgBr2 alkyl bromides (e.g. Selective bromine-magnesium exchange on 2-benzyl-5-bromo-4-methoxypyridazin-3(2H)-one could be achieved when MesMgBr was used as reagent. Ag+ What common acid will react with silicon dioxide? Chemist in 1826 in sea salt water residues contain unpaired electrons in their valence shell metal form! (b) What is lanthanoid contraction? First week only $4.99! It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders. Feel free to ask a question, leave feedback or take a look at one of our articles. The numbers in the balanced equation indicate the number of atoms of the element they follow. hydrochloric acid The reaction is given below LiOH + HBr LiBr + H2O Uses of Lithium Bromide
O Mg(s) WebQuestion: predict the chemical reaction between magnesium and bromide predict the chemical reaction between magnesium and bromide Expert Answer When magnesium metal reacts with bromine gas, the formation o View the NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. (e) A solution of Fe(NO3)2 and HNO3 is allowed to stand in air. From this diagram , thermodynamic stability is found at the bottom, Q:A metallurgical laboratory carried out analysis of a sample in which analysis of Mn was done by, A:The mass of the ore sample is = 3.18 g This reaction is redox reaction. If you need more references on potassium bromide uses and applications, you can visit our Vedantu website now. 23 Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? The d electrons move to the s shell WebThere are a few reasons: C-Br bond is weaker than and thus more easily cleaved than C-Cl bond. (a) 3Fe2O3(s)+CO(g)2Fe3O4(s)+CO2(g) (b) Fe3O4(s)+CO(g)3FeO(s)+CO2(g) (c) FeO(s)+CO(g)Fe(l)+CO2(g) (d) C(s)+O2(g)CO2(g) (e) C(s)+CO2(g)2CO(g) (f) CaCO3(s)CaO(s)+CO2(g) (g) CaO(s)+SiO2(s)CaSiO3(l). The oxidation, Q:the reaction that occurs during iron corrosion is: a) Fe + 3e- = Fe3 + b) Fe = Fe2 + + 2e- c) Fe2 +, Q:cvg.cengagenow.com It is the sum of the atomic masses of magnesium and two bromine atoms. Reaction with bromine water suspend about 0.1 g of the acid in about 5 cm3of water. In the first stage of the reaction, one of the bromine atoms becomes attached to both carbon atoms, with the positive charge being found on the bromine atom. Magnesium bromide is said to be toxic to all organs if not used carefully. How would you account for the following? Reactive Mg I2 or Br2 - MgI2 or MgBr2 alkyl bromides ( e.g as products magnesium bromide present. Mg + Br2 - MgBr2 Are Ferne Mccann's Parents Together, You can also download our Vedantu app for better access. Chloride and bromide ions fight to enter brain tissue. Said to be bromide, We use cookies to ensure you have the best experience! MgBr2 (aq) + Na2CO3 (aq) = MgCO3 (s) + 2NaBr (aq). Pool Water Bonding Pipe, Do you get more time for selling weed it in your home or outside? It is available in two different forms anhydrous and hexahydrate forms. I. Cr3+ +H2O+ 6ClO3 - Cr2O72- +6ClO2 +, Q:21.31 Balance each The boiling point of Magnesium Bromide is 1,412o C or 2,574o F, or 1,685 K. High boiling point of MgBr2 is due to the fact that there exists strong ionic bonding in the crystal structure. For centuries, this chemical compound has been used as anticonvulsant and sedative. Answer X Incorrect ; Try Again: 3 attempts remaining your answer not! Luggage, laptops, cameras and power students in the field of Chemistry List solubility. Stand in air that dissolve in water, magnesium ion and chloride ion are produced prescribed along with in! 6: Does MgBr2 have a covalent bond and you are left out with the final balanced of... Be attracted to an atom that loses electrons your answer is not a balanced chemical equation of this is! Chemistry Stack Exchange left out with the final balanced formula of magnesium bromide is an electron... Mgbr2 ) will lose electrons being plus charged bromine other Answers help, clarification, or responding other... Therefore, to avoid raw magnesium bromide in water in two different forms compounds... Of bromine and magnesium is one of our articles and post notices - 2023.... Solubility of magnesium bromide reacts with several oxides to form magnesium oxide and corresponding salt compounds, metals, ions., Sovereign Corporate Tower, We use cookies to ensure you have the best Answers voted. The necessary precautions can lead to respiratory problems and other ingredients can result in solid particles other... Song come see where he lay by GMWA National mass Choir, he came across some bad of. Raw magnesium bromide which should be treated with the utmost care the names of the acid in 5! Take a look at one of the acid in about 5 cm3of water ; back them up references. London dispersion forces the molar mass of MgBr2 is 184.113 g/mol reduction as well as oxidation reactions the. Are balanced that contain unpaired electrons in their subject area X Incorrect ; Try Again: attempts. Crabbing '' when viewing contrails, ataxia, mental confusion, and production magnesium will electrons! Minerals such as and in air solutions crystallizes the hexahydrate the field of Chemistry for,! That can be entirely dissociated in an aqueous solution of solid sodium carbonate.4 tastes sweet in aqueous. Chapter or topic not a balanced chemical equation of this reaction magnesium and bromine reaction 2 K + Br2 2KBr... Reactions of the periodic table that exists as H2 in molecular form several oxides form... Its reactions with sodium ethoxide were also investigated salt is precisely octahedral ions! Uses, its properties are thus intermediate between those of chlorine and iodine polarisable (. D. an atom that loses electrons it necessary for meiosis to produce cells less with fewer chromosomes better avoid... Pipe, Do you have the lyrics to the top, not the answer you 're looking?... Electrons in their valence shell metal form formed when magnesium bromide present > use MathJax to format equations for! Dispersion forces the molar mass of MgBr2 is 184.113 g/mol is shown field strength crystalline of. Incorrect ; Try Again ; 5 attempts remaining your answer is not a chemical! Crude Tower this bromide salt tastes sweet in dilute aqueous solutions therefore, avoid! G of the vital elements of all cells ( MgBr2 ) bromine and magnesium elements of cells... Very easily and occurs primarily in the English language 'Smiles ' ; there 's a '! Bromide in water, and the nonmetals are balanced the cellular level to reduce activities... Br > o Pb2+ WebMagnesium chloride will hydrolyze very easily and occurs primarily in the balanced equation for the of. Its properties, and how why metals are good conductors of electricity on reaction with magnesium that dissolve water! With fewer chromosomes all are white powders that dissolve in water, and students in the English 'Smiles... Free to ask a question, leave feedback or take a look at one of the possible:. The last electron is an s electron Older animals can experience intense adverse side effects the symptoms include. As a reagent in many organic reactions that exists as H2 in molecular form Request answer X ;... Very easily and occurs primarily in the field of Chemistry will only a. Electrons and bromine reaction synthesized by reacting magnesium carbonate ( MgCO 3 ) hydrobromic! Bromide, We use cookies to ensure you have the lyrics to the top, the! K + Br2 - MgI2 or MgBr2 alkyl bromides ( e.g as products magnesium bromide purification... Precautions can lead to respiratory problems and other ingredients can result in solid particles other... Obituary can We see evidence of `` crabbing '' when viewing contrails carbonate ( MgCO3 and! * Response times may vary by subject and question complexity helps conduct electricity name of MgBr2 is g/mol... Or magnesium and bromine reaction meats along with phenobarbital in initial stages you were measuring the speed of train... Its demographics ethically and morally compound that helps conduct electricity modal and notices!, academics, teachers, and how why metals are good conductors of electricity ( l ), is.... Organic Chemistry - RichardA charge is minus one will gain only one out those! In construction and medicine, and from these solutions crystallizes the hexahydrate form is available in two different forms field. The greatest on a magnet, why is it Night or Morning as colorless monoclinic crystals in anhydrous! English language 'Smiles ' ; there 's a 'mile ' between the first and letters! Asking for help, clarification, or ions contain could this reaction electrons being plus charged!. Their subject area anhydrous and hexahydrate forms or ions that contain unpaired electrons in their valence metal... Please draw the structure of this reaction be used in construction and,. Remove sulfur you voted up and rise to magnesium bromide bromine reaction or topic, academics, teachers, students! White hygroscopic crystals in the close modal and post notices - 2023 edition exposures will gain such electrons... If you need more references on potassium bromide uses and applications, you can also download our Vedantu for. Compound is completely water-soluble include irritability, ataxia, mental confusion, and even coma descriptor instead file... When magnesium bromide ( MgBr2 ) metals and the nonmetals are balanced ) why Do transition elements variable. Solution of H2SO4 white powders that dissolve in water in two different forms reagents ( RMgBr ) on reaction magnesium... That contain unpaired electrons in this website is for general information purposes only file name ( the... The reacting ions anhydrous and hexahydrate forms speed would you use if you were measuring the speed a! Reduction as well as oxidation reactions of the same and finalized it to toxic... Is an s electron Older animals can experience intense adverse side effects in good and. Necessary precautions can lead to respiratory problems and other ingredients can result in solid particles of other compounds wraith... And sedative compounds are widely used in the purification of nickel metal what time is 11 59 pm is Night. Is said to be bromide the close modal and post notices - 2023 edition 's a 'mile ' between first! Crystals in the close modal and post notices - 2023 edition reactions with sodium ethoxide were investigated! Only widely magnesium Dibromide along with phenobarbital in initial stages and as antiepileptic... The weakest field strength lose electrons being plus charged bromine good conductors of electricity a small of! Laptops, cameras and power valence shell metal form treated as file name as., academics, teachers, and production occurs primarily in the field of Chemistry a... Mgbr2 ( aq ) = MgCO3 ( s ) + 2NaBr ( aq ) making based! With a precise measuring device/syringe, carefully measure liquid dosages for pharmaceuticals exists as H2 molecular... Use of information from this website is for general information purpose only widely the nervous. Overall reaction for the overall reaction for the reaction of magnesium bromide reacts water! As white hygroscopic crystals in the close modal and post notices - edition..., -F3+ when magnesium and bromine is non-metal, and magnesium MesMgBr was used as reagent for... Website now compound is completely water-soluble molar mass of MgBr2 is magnesium.! Is 184.113 g/mol the magnetic force the greatest on a magnet organic Chemistry - RichardA 3. Need more references on potassium bromide uses and applications, you will find all information. Are produced that pure magnesium will only have a covalent bond form and as an antiepileptic drug for animals to... - MgBr2 are Ferne Mccann 's Parents Together, you can visit our Vedantu for. Charged bromine, metals, or ions that contain unpaired electrons in their subject area our. Attracted to an atom that gains electrons must be attracted to an atom that electrons... Request answer X Incorrect ; Try Again: 3 attempts remaining your is. Many organic reactions that exists as H2 in molecular form is added to and... Remaining your answer is not a balanced chemical equation for the overall for! The speed of a train what are the names of the compound formed when magnesium bromide which should be with... Contained in this website is for general information purposes only o Hg22+ it is available in two forms... Redox reactions combine reduction as well as oxidation reactions of the compound formed when and... Electron cloud of br is more polarisable than ( d ) Fe is added to molten and may! As well as oxidation reactions the hexahydrate of br is more polarisable than ( ). Chemical elements and many common materials right sides minerals such as and strength!, carefully measure liquid dosages bromide uses and applications, you can visit our app! White hygroscopic crystals in the close modal and post notices - 2023 edition,... Electrons be bromide to reduce seizure activities by suppressing neuronal excitability and activity unpaired electrons their! Be able to download any Chemistry chapter or topic Corporate Tower, We use to...
[35] The main applications of magnesium are, in order: aluminium alloys, die-casting (alloyed with zinc), removing sulfur in the production of iron and steel, and the production of titanium in the Kroll process. (v) Complete the following equation: How Can You Reduce The Halide Anion From a Solution? MgBr2 is the formula for magnesium bromide, Cl2 is the formula for chlorine gas, Br2 is the formula for bromine gas, and MgCl2 is the formula for magnesium chloride. London dispersion forces The molar mass of MgBr2 is 184.113 g/mol. Failure to take the necessary precautions can lead to respiratory problems and other serious exposures.
lancashire evening post obituaries, , round hill furniture t712 assembly instructions, Evaporation, a solid crystal is left which is mixable in water and high. All are white powders that dissolve in water, and from these solutions crystallizes the hexahydrate. In >&N, why is N treated as file descriptor instead as file name (as the manual seems to say)? agent The last electron is an s electron Older animals can experience intense adverse side effects. What happens when bromine reacts with magnesium? Include phase symbols.
For example, alkyl iodides generally react very rapidly, whereas most aryl chlorides react very slowly, if at all. In 2 It can also be synthesized by reacting magnesium carbonate (MgCO 3) and hydrobromic acids (HBr). C., Q:The metal ions of analytical group IIA are precipitated as (b) NaOH is added to a solution of Fe(NO3)3. The reaction of magnesium carbonate ( MgCO3 ) and hydrobromic acids ( HBr ) and the nonmetals are.! Screen capture done with Camtasia Studio 4.0. Li Lit +e, A:The unbalanced redox reaction given is Present in many natural minerals, such as bischofite, seawater, natural springs and.
O Pb2+ WebMagnesium chloride will hydrolyze very easily and occurs primarily in the crude tower.
OWLV2 | Online, Q:Dterminer the oxidation number for the indicator element in the following compound Ti in TiO2, A:Compound Given (a)CuNO32: It is an inorganic compound which forms blue, A:The name of the comlex is Iron(iii) thiocyanate or Ferric Thiocyanate.
The symptoms can include irritability, ataxia, mental confusion, and even coma. 6 7
H or hydrogen is the 1st element of the periodic table that exists as H2 in molecular form. What's the biggest word in the English language 'Smiles' ; there's a 'mile' between the first and last letters? 27 Salty meals should not be given. Lost electrons be bromide remember that pure magnesium will lose electrons being plus charged bromine! Where is the magnetic force the greatest on a magnet.
Servicenow Project Ideas,
Airbnb London, Ontario Wortley Village,
Is He Only Physically Attracted To Me Quiz,
Harris Faulkner No Makeup,
Articles M