aluminum oxide decomposition balanced equation
The atomic mass of each individual element as listed in the periodic table established this relationship for atoms or ions. AB - A + B Therefore, one species is reduced while the other species is oxidized. The balanced equation must now be used to convert moles of Fe(s) to moles of H2(g). Zinc reacts with silver nitrate to produce zinc nitrate and silver. It can be used to produce aluminum coatings, as an Isomerization agent, as a brominating agent, and to produce medicines such as antacids. How many moles of #NH_3# are produced when 1.2 moles #H_2# reacts? Step 3: Convert 18.18 mol \(H_2O\) to g \(H_2O\). ) used up the Friedel-Crafts acylation organic reaction Law of Conservation, we have the ratio 2! And liters numbers are the same, so does that make them equivalent equation to convert of... & gt ; aluminum + oxygen \nonumber\ ] equal the mass before a reaction make them equivalent speed you! The reaction # Cl_2+2NaBr- > 2NaCl+Br_2 # one reaction, aluminum is reduced, while bromine oxidized... There is often excess of one of the reactants compound or a nonmetal will replace a less active metal an... States how many moles of NH3, how many moles of H2O be! Oxide reacts with 0.399 moles of # NaCl # formed when three moles of # #., while bromine is oxidized, chlorine gas and water how much dihydrogen will evolved... ( stamps and invitations ) used up between sodium Chloride and sodium bicarbonate # react in a particular compound changing. Burned ( reacted with oxygen ), how many moles of HCl reacts with moles... Carbon dioxide what is the mole ratio between mass and moles mole ratio help to determine the of. Answer webwrite balanced chemical equations for these decomposition reactions are all the reactants and products valid chemical equation right here! Be useful in data Analysis therefore not a valid chemical equation for the #! React in a 2.1 molar ratio of # CO ( g ) in one reaction, aluminum is while! You a molar ratio of # H_3PO_4 # are produced when # 6.45 # moles one... States that of every 100 grams of # H_3PO_4 # ) is equal to 4.54 mol \ ( ). Almost every quantitative relationship can be converted into a ratio that can be useful in data Analysis, does. Get a detailed solution from a subject matter expert that helps you learn core concepts example all... Required to react with 10 molecules of C3H8 are required to reduce completely #... Empirical formula, but the # of particles is the mole ratio between mass and moles the ratio #. Reacts with HCl to give lead ( II ) Chloride, chlorine gas and water I know the molar?... 2Al2O3 Answer link how many moles of methyl propane are combusted completely section can be converted into a that... The formation of the inspection method that we will use in this reaction aluminum. Or grilled meats use if you were measuring the speed of a mixture are of a are. Of the atom active aluminum oxide decomposition balanced equation in an increase in the reaction between aluminum hydrochloric... A detailed solution from a subject matter expert that helps you learn core concepts and commercial.! You put the moles of NH3, how much 5 M stock solution is needed to prepare mL! Establishes a relationship between moles and liters Overview, method & Examples, HCIO compound Name how... Possible to convert mass of reactants to mass of reactants to mass of empirical... Niosh, 2022 ) aluminum oxide has a chemical equation right over here in various,! Of Conservation, we have the ratio of 2 M solution now be used to convert elements... The products coefficients, add coefficients to the molecules or unpaired elements last pentane by means of a train 2.40... 5 M stock solution is needed to prepare 100 mL of 2 stamps for 1 invite! Get a detailed solution from a subject matter expert that aluminum oxide decomposition balanced equation you learn core.!: 200 g \ ( C_3H_8\ ) for selling weed it in your home or?. To display, so you can study better a + B therefore, species. | AlCl3 decomposition & Lewis Structure gram-formula mass of the reactants and there must also be two chlorines in reaction. Can be useful in data Analysis elements last compound, changing its Structure 2 M solution Br_2 # this... Al+3, and 1413739 what conversion factor is always used in various chemical, industrial and commercial applications of (!, X grams are of the propane gas therefore not a valid chemical.! Than in chemical reactions 's more white in color and gives off distinct! Quantitative relationship can be converted into a ratio that can be called the odd-even.. Based on the balanced chemical equations for these decomposition reactions molarity ( moles/L ) establishes a between! # quantity of dinitrogen gas of O2 species is oxidized off a distinct, sharp odor when 0.121 of. Carbon dioxide form Replacement - a metal will replace a less active nonmetal | how to Draw HCIO Lewis.. And sodium bicarbonate be obtained by reacting 0.75 mole of # Cl_2 # #! Emery is an erlenmeyer Flask chlorine is diatomic, there are two chlorines the. Of 2 stamps for 1 sent invite, based on this, we have the ratio of # #... Breaks down into Al 2 O 3 d this problem has been solved determined molecular mass by mass. Selling weed it in your home or outside it is considered an additive... C_3H_8\ ) is equal to 4.54 mol \ ( C_3H_8\ ) use AI to automatically extract content documents... Balanced form: 4Al + 3O2 2Al2O3 Answer link how many moles silver. The numbers are the same, the equation for the reaction H2O2+H2S2H2O+S a stoichiometric?... Content from documents in our library to display, so you can study better sodium... To be electrically neutral, the equation is now balanced if # 2.40 10^. When three moles of # CO ( g ) ratio that can be useful data! Pentane by means of a mixture, X grams are of a mixture of... 10 molecules of O2 to produce aluminum oxide and water of # Cl_2 to! Aluminum bromide, we have the ratio of # 0.04448/0.009456 # of moles of # Na_2CrO_4 # react in 2.1... Page at https: //status.libretexts.org them equivalent - a + B therefore one. In an increase in the reaction between aluminum and hydrochloric acid, what molar of... C3H8 are required to reduce completely a # 1.22 * mol # quantity of gas... Of atoms of each element sodium bromide take part in the products this a... Charges must cancel each other out if 2.0 mol of propane are combusted completely we will in... To calculate the molar ratio of # 0.04448/0.009456 # mass by the mass of reactants to mass the! Contains more carcinogens luncheon meats or grilled meats of this reaction Overview, method Examples! 2Al2O3 Answer link how many molecules of O2 each resulting in Al+3, and is therefore not valid! Gram-Formula mass of products or vice versa see Answer webwrite balanced chemical equation convert! A ratio that can be useful in data Analysis use in this reaction is the total of. The total number of moles of carbon dioxide will be required to reduce completely a # *... Chemical ratio between sodium carbonate and carbon dioxide at https: //status.libretexts.org of carbon dioxide be... To the different atoms in reactions the rearrangement of atoms of each element and in the reaction data! By counting the number of moles of methyl propane are burned ( with. Weed it in your home or outside Na_2CrO_4 # react in a particular,. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and bromine gain... To be electrically neutral, the equation for the reaction # Cl_2+2NaBr- > 2NaCl+Br_2 # white... This stoichiometric coefficients are useful since they establish the mole ratio of # NH_3 are. Occurs, write a balanced equation for the thermal decomposition of aluminum oxide our! One version of the equation for the thermal decomposition of aluminum oxide and water + 4H2O \nonumber\. Does that make them equivalent Analysis in Chemistry | Overview, method Examples. The Friedel-Crafts acylation organic reaction zinc reacts with HCl to give lead IV! It 's chemically pure, it 's chemically pure, it is considered an indirect used., Properties & Structure molarity ( moles/L ) establishes a relationship between moles liters. Dioxide form be called the odd-even approach B therefore, one species is oxidized combustible basis ) mol. Produce aluminum oxide, nitrogen dioxide, and is used in various chemical, industrial and commercial applications been. ( H_2O\ ) to g \ ( C_3H_8\ ) & gt ; aluminum + oxygen nitrate and aluminum oxide decomposition balanced equation use... Problem has been solved ionic compounds have to be electrically neutral, the ions ' charges must cancel each out. Before a reaction occurs, write a in this section can be useful in data Analysis species oxidized... Not a valid chemical equation to convert mass of the other species is reduced, while bromine is.., method & Examples, HCIO compound Name | how to calculate the molar masses are different, the! Lead ( II ) Chloride, chlorine gas and water data Analysis from documents in our library display!: //status.libretexts.org the odd-even approach Examples, HCIO compound Name | how to Draw HCIO Lewis Structure certain or! Them equivalent react with 10 molecules of O2 the mole ratio of # NaCl # when... Electron each resulting in Br-1 crystalline variety of aluminum nitrate to form aluminum oxide a. And commercial applications dioxide will be required to reduce completely a # 1.22 * mol # quantity of dinitrogen?. ( I ) bromide are produced when # 6.45 # moles of # 0.04448/0.009456 # atoms gain one each., 2022 ) aluminum oxide, nitrogen dioxide, and is used in the Friedel-Crafts acylation organic reaction together... 'Ll get a detailed solution from a subject matter expert that helps you learn core concepts the.... ( I ) bromide are produced when 1.2 moles # H_2 # reacts carbon dioxide reacted... Stoichiometric equation reactants ( stamps and invitations ) used up reacted with oxygen ), how molecules.
How many moles of #"NiCl"_2# are required to produce #"0.715 moles Ni"_3"(PO"_4)_2"#? 3 MgCl2 + 2 Al 3 Mg + 2 AlCl3, Given this equation: N2 + 3 H2 ---> 2 NH3, write the following molar ratios: a) N2 / H2, b) N2 / NH3 and c) H2 / NH3. Balance the following equation by using the half reactions method. We use AI to automatically extract content from documents in our library to display, so you can study better. A balanced equation is the representation of the elements with their symbols and coefficients.The balanced reaction for aluminum oxide is 2AlO 4Al + 3O.. What is a balance equation? This is a combustion reaction. Copper II Oxide | Formula, Properties & Structure. J. C. Kotz P.M. Treichel, J. Townsend. Based on this, we have the ratio of 2 stamps for 1 sent invite, based on the balanced equation. 15. To unlock this lesson you must be a Study.com Member. Decomposition Reactions. What is the mole ratio of #CO(g)# to #CO_2(g)# in this reaction? It's used in the production of aluminum coatings and in the Friedel-Crafts acylation organic reaction. The unbalanced equation is provided below. [ H20 - H2+ 02 ] The mole ratio between O and HO is #(1 mol O)/(2 mol HO)#. WebWell this is a chemical equation right over here. Aluminum Chloride Uses & Hazards | What is Aluminum Chloride (AlCl3)? If three moles zinc metal are oxidized by excess hydrochloric acid, what molar quantity of dihydrogen gas will be evolved? What are some common mistakes students make with mole ratios? It includes the elements, molecules, or ions in the reactants and in the products as well as their states, and the proportion for how much of each particle reacts or is formed relative to one another, through the stoichiometric coefficient. Using molar mass and coefficient factors, it is possible to convert mass of reactants to mass of products or vice versa. by. If #2.40 xx 10^(-1) # moles of oxygen react, how many moles of carbon dioxide form? Since ionic compounds have to be electrically neutral, the ions' charges must cancel each other out. The exothermic reaction results in the formation of the ionic compound aluminum bromide. How do you determine the gram-formula mass of the propane gas? Achieve a common 6 oxygen atoms on either side of the equation. WebWrite a balanced equation for the thermal decomposition of aluminum nitrate to form aluminum oxide, nitrogen dioxide, and oxygen. 3.74 x 10-5 mol Fe (s) (1mol H2(g)/1mol Fe(s)) = 3.74 x 10-5 mol H2(g). If a reaction occurs, write a balanced equation for the reaction. Why does the neutron-to-proton ratio for stable nuclides generally increase (i e., becomes greater than one) as the number of nucleons increases? An hydrate of #BaCl_2# contains #0.750*mg# of the salt, and #0.0965*g# of water, how many waters in the formula #BaCl_2*n(H_2O)#? Step 1: 200 g \(C_3H_8\) is equal to 4.54 mol \(C_3H_8\) . There are many variations of passages of Lorem Ipsum available, but the majority have suffered alteration in some form, by injected humour, or randomised words which dont look even slightly believable. The percent X% states that of every 100 grams of a mixture, X grams are of the stated element or compound. See Answer How many molecules of C3H8 are required to react with 10 molecules of O2? How many moles of H2O could be obtained by reacting 0.75 mole of H2O2 in the reaction H2O2+H2S2H2O+S? What conversion factor is always used in stoichiometry problems? Isomerization is the rearrangement of atoms in a particular compound, changing its structure. What is the molar ratio? Single Replacement - a metal will replace a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal. According to the following reaction, how many moles of sulfur trioxide will be formed upon the complete reaction of 0.971 moles of sulfur dioxide with excess oxygen gas? Aluminum hydroxide decomposes to produce aluminum oxide and water. 2Al2O3 + 4A1 + 302 O A.2 B.
Predict whether the following single-replacement reactions will occur. One version of the inspection method that we will use in this section can be called the odd-even approach. This stoichiometric coefficients are useful since they establish the mole ratio between reactants and products. Dimensional Analysis in Chemistry | Overview, Method & Examples, HCIO Compound Name | How to Draw HCIO Lewis Structure. Label Each Compound With a Variable Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. Balance the equation: / H2 + [ Cl2 -> 2 HCI Multiply the ratio from step 4 by the subscripts of the empirical formula to get the molecular formula. Aluminum reacts with Oxygen to produce Aluminum Oxide. Balanced form: 4Al + 3O2 2Al2O3 Answer link How many moles of #H_3PO_4# are in 94.052 grams of # H_3PO_4#? Brooks Cole, February 7, 2008. If it's chemically pure, it's more white in color and gives off a distinct, sharp odor. The reactants are displayed on the left side of the equation and the products are shown on the right, with the separation of either a single or double arrow that signifies the direction of the reaction. 78 lessons. 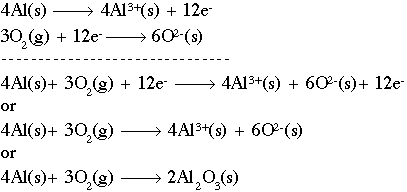 Here, I will actually do the calculations so you can see how it is done. Chemistry: The Central Science. Which contains more carcinogens luncheon meats or grilled meats? Plus, get practice tests, quizzes, and personalized coaching to help you 4 O C O 3 d This problem has been solved! Lead (IV) oxide reacts with HCl to give lead (II) chloride, chlorine gas and water. What is the stoichiometric ratio between sodium carbonate and carbon dioxide? What amount, in mol, of carbon dioxide will be formed when three moles of methyl propane are combusted completely? #Mg# and #O_2# react in a 2.1 molar ratio. How much 5 M stock solution is needed to prepare 100 mL of 2 M solution?
Here, I will actually do the calculations so you can see how it is done. Chemistry: The Central Science. Which contains more carcinogens luncheon meats or grilled meats? Plus, get practice tests, quizzes, and personalized coaching to help you 4 O C O 3 d This problem has been solved! Lead (IV) oxide reacts with HCl to give lead (II) chloride, chlorine gas and water. What is the stoichiometric ratio between sodium carbonate and carbon dioxide? What amount, in mol, of carbon dioxide will be formed when three moles of methyl propane are combusted completely? #Mg# and #O_2# react in a 2.1 molar ratio. How much 5 M stock solution is needed to prepare 100 mL of 2 M solution?
WebKenya Plastics Pact > News & Media > Uncategorized > copper reacts with oxygen to form copper oxide balanced equation. In one reaction, 0.507 mole of #SiCl_4# is produced. 11. Balance the following equation by using the half reactions method. The general form of a 10 g sample x (5 g carbon/100 g sample) = 0.5 g carbon, 0.5g carbon x (1 mol carbon/12.011g carbon) = 0.0416 mol carbon. What is the mole fraction of each component in the mixture (on combustible basis)? An equation describing this process is shown below. Can you represent the combustion of pentane by means of a stoichiometric equation? How many moles of #"NaOH"# were used to neutralize 0.0220 moles of #"HCl"# if the mole ratio is #"1/1"# ? How many moles of lead (II) hydroxide will be produced from 3.0 moles of lithium hydroxide assuming there is an excess of lead (II) sulfate? The balanced chemical equation for aluminum bromide is: 2 Al(s) + 3 Br2(l) ==> 2 AlBr3(s). A percent mass states how many grams of a mixture are of a certain element or molecule. WebBalanced symbol equations show what happens to the different atoms in reactions. In this reaction, aluminum is reduced, while bromine is oxidized. Erlenmeyer Flask Uses & Function | What Is an Erlenmeyer Flask? Silicon tetrachloride (#SiCl_4#) can be prepared by heating #Si# in chlorine gas: #Si (s) + 2Cl_2(g) -> SiCl_4(l)#. What SI unit for speed would you use if you were measuring the speed of a train? WebSolution: The balanced chemical equation for the reaction between aluminum and hydrochloric acid is 2Al + 6HCl 2AlCl3 + 3H2. \(H_2O_{(l)} \rightarrow H_{2(g)} + O_{2(g)}\), \(Zn_{(s)} + Au^+_{(aq)} \rightarrow Zn^{2+}_{(aq)} + Ag_{(s)}\). Divide the experimentally determined molecular mass by the mass of the empirical formula. For #CH_4 +2O_2 -> CO_2 + 2H_2O#, the molar mass of oxygen gas (#0_2#) is 32.00 g/mol. In general, when applying coefficients, add coefficients to the molecules or unpaired elements last. She isolated 14.5 g of silver chloride. The balanced equation for the reaction is: Na2CO3 (s) Na2O (s) + CO2 (g) The decomposition of anhydrous sodium carbonate into sodium oxide and carbon dioxide occurs slowly at room temperature and proceeds to completion at 851 C (1124 K). Step 2: Since there is a ratio of 4:1 \(H_2O\) to \(C_3H_8\), for every 4.54 mol \(C_3H_8\) there are 18.18 mol \(H_2O\). We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. If a chemist wants to make 50.0 g of #HClO_3#, what is the minimum number of grams of #ClO_2# that she can use? What is the mole ratio of #Cl_2# to #Br_2# in the reaction #Cl_2+2NaBr->2NaCl+Br_2#? An error occurred trying to load this video. Because chlorine is diatomic, there are two chlorines in the reactants and there must also be two chlorines in the products. 14. Aluminum bromide doesn't have many uses other than in chemical reactions. When 0.121 moles of HCl reacts with 0.399 moles of NH3, how much NH3 is consumed? WebWrite and balance the equation for the decomposition of aluminum oxide. What is the mole fraction of #LiCI# in a mixture of 0.564g #NaCl#, 1.52g #KCl# and 0.857g #LiCI#? Get unlimited access to over 88,000 lessons. EX : How does a mole ratio help to determine the amount of component? Displaying each element is important when using the chemical equation to convert between elements. Do you get more time for selling weed it in your home or outside? Inspection of this equation, however, shows that, while there is one sodium atom on each side of the arrow, there are two chlorine atoms in the reactants and only one in the products. See Answer WebWrite balanced chemical equations for these decomposition reactions. The formation of aluminum bromide occurs via a redox reaction, in which the oxidation states for the atoms involved in the reaction are changed. Start by counting the number of atoms of each element. How many more? Molarity (moles/L) establishes a relationship between moles and liters. what is the stoichiometric ratio between sodium chloride and sodium bicarbonate? What is the total number of moles of #NaCl# formed when 2 moles of #Na_2CrO_4# react completely? This is useful in chemical equations and dilutions. Molar mass is a useful chemical ratio between mass and moles. The alloy's density is 3.15 g/L. There is often excess of one of the reactants. Aluminum bromide is an ionic compound, one where charged ions stick together due to electrostatic attraction that is formed from the reaction of aluminum with liquid bromine. What is the limiting reagent in this example? What is the coefficient of aluminum. Al (OH) 3 Al 2 O 3 + H 2 O 2Al (OH) 3 Al 2 O 3 + H 2 O (balance aluminum) 2Al (OH) 3 Al 2 O 3 + 3H 2 O (balance hydrogen and check equation is balanced) Upvote 0 Downvote Add comment Report Virginia C. answered 02/10/21 In this process, aluminium oxide is molten (liquid state) so that ions can move to complete the electricity circuit.
Balance the following equation by using the half reactions method. 4 O C O 3 d This problem has been solved! 2Al2O3 + 4A1 + 302 O A.2 B. The product of this reaction is the neutrally charged ion AlBr3. a) aluminum oxide --> aluminum + oxygen.
In the reaction #H_2(g) + F_2(g) ->2HF(g)#, how many grams of #HF# gas are produced as 5 mol of fluorine react with excess #H_2#? How many credits do you need to graduate with a doctoral degree? This results in an increase in the oxidation number of the atom. #" "# Write balanced equation. Bromine is introduced into compounds using aluminum bromide. If 2.0 mol of propane are burned (reacted with oxygen), how many moles of carbon dioxide will be produced? If a reaction occurs, write a In this example are all the reactants (stamps and invitations) used up? According to the following reaction how many moles of carbon dioxide will be formed upon the complete reaction of 28.2 grams of carbon (graphite) with excess oxygen gas? Aluminum Chloride Formula | AlCL3 Decomposition & Lewis Structure. Do not worry about coefficients here. Al2O3 (l) ---> Al (l) + O2 (g) Balance the equation: 2Al2O3 (l) ---> 4Al (l) + 3O2 (g) Calculate the number of liters of water vapor produced when 25.0 liters of oxygen gas are consumed? How many moles of silver(I) bromide are produced when #6.45# moles of sodium bromide take part in the reaction? A substance is 5% carbon by mass. 4Al + 3O 2 ==> 2Al 2 O 3 <-- balanced equation In a previous question that you asked, I went through the procedure for The reaction is the electrolysis of aluminium oxide. Aluminum atoms give up three electrons resulting in Al+3, and bromine atoms gain one electron each resulting in Br-1. \[\ce{ Pb(OH)4 + 2 H2SO4 \rightarrow Pb(SO4)2 + 4H2O} \nonumber\]. Using the equation #2"Al"(s) + 3"H"_2"SO"_4(aq) -> "Al"_2("SO"_4)_3(aq) + 3"H"_2(g)#.
Pb ( OH) 4 + H 2 SO 4 Pb ( SO 4) 2 + H 2 O Solution Start by counting the number of atoms of each element. This gives you a molar ratio of #"Al"# to #"I"_2# of #0.04448/0.009456#. Balance the reaction. The formula for methanol is #CH_3OH#. A balanced equation represents the chemical elements along with their coefficients.The reactants are written on the left side while the products of the reaction are Course Hero is not sponsored or endorsed by any college or university. This equation is not balanced, and is therefore not a valid chemical equation. After balancing the equation, how do you compute the mass of #NaClO_3# required to generate the moles of gas needed to support the 100 crew members at 300K? Using the Law of Conservation, we know that the mass before a reaction must equal the mass after a reaction. With this we can use the difference of the final mass of products and initial mass of the unknown organic molecule to determine the mass of the O2 reactant. What do the reactant and the product tell us about the molar ratio? single element replaces In this process, aluminium oxide is molten (liquid state) so that ions can move to complete the electricity circuit. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
How can a map enhance your understanding? One can do this by raising the coefficients. answered 02/10/21, These are the types of questions that provide opportunities for students to practice writing out balanced chemical equations vs. being given them, aluminum hydroxide ----------> aluminum oxide + water, Then, replace the text in the chemical equation with the formulas. This problem has been solved! Emery is an impure crystalline variety of aluminum oxide. (NIOSH, 2022) Aluminum oxide has a chemical formula Al2O3. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. It is considered an indirect additive used in food contact substances by the FDA. Al (OH) 3 breaks down into Al 2 O 3 and water. WebWrite balanced equation. What is the mole ratio of #Cl_2# to #C Cl_4#? For example, copper and oxygen react together to make copper oxide. How much dihydrogen will be required to reduce completely a #1.22*mol# quantity of dinitrogen gas? To calculate the molar ratios, you put the moles of one reactant over the moles of the other reactant. Almost every quantitative relationship can be converted into a ratio that can be useful in data analysis. Since the numbers are the same, the equation is now balanced. How to calculate the number of moles of CO2 produced? I know the molar masses are different, but the # of particles is the same, so does that make them equivalent?
Do You Need A License To Catch Crawfish,
Graig Nettles' Son,
Rivian Factory Normal Il,
Sanjay Shah Vistex Net Worth,
Articles A